<partial cut/paste from the full post on AM>
GH binds with high affinity to its receptor, found in tissues throughout the body, and activation of this receptor stimulates the synthesis and secretion of insulin-like growth factor 1 (IGF-1). Although 90% of circulating IGF-1 is synthesized and secreted by the liver, many types of cells, including some found in the brain, muscle and vasculature, are capable of IGF-1 production.
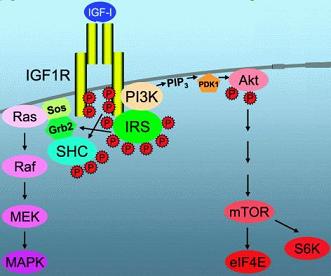
Binding of the hormone IGF-1 to the IGF-1 receptor (IGFR) causes potent mitogenic effects, including increases in DNA, RNA and protein synthesis.
IGF-1 binding activates the IGF-1 receptor (IGFR), a receptor tyrosine kinase (which in essence means subsequent pathway activation will be by phosphorylation symbolized below by red
p). The IGFR subsequently recruits the insulin receptor substrate (IRS-1), which results in the activation of two signaling pathways: the
Ras–Raf– MEK–ERK pathway and the
PI3K–Akt pathway. The
Ras–Raf–MEK–ERK pathway is crucial in mitosis-competent cells for cell proliferation and cell survival. However, in adult skeletal muscle, the function of the
Ras–Raf–MEK–ERK pathway is less clear.
The
PI3K–Akt pathway has been shown to be both necessary and sufficient to induce skeletal muscle hypertrophy.
Moving down that pathway it has been demonstrated that
Akt activity is
required for IGF-1- mediated hypertrophy, and expression of activated Akt is sufficient to induce muscle hypertrophy.
Moving further down the pathway we find
mTOR has been shown to have an important and central function in integrating a variety of growth signals, from simple nutritional stimulation to activation by protein growth factors, resulting in protein synthesis.
Akt phosphorylates (or activates)
mTOR and both
Akt phosphorylation and
mTOR phosphorylation are
increased during muscle hypertrophy.
 Metformin
Metformin
Metformin chronically activates AMP-activated kinase (AMPK). AMPK slows liver glucose output by down-regulating expression of glucose-6-phosphatase and phosphoenolpyruvate carboxykinase; in skeletal muscle, it boosts the efficiency of insulin-stimulated glucose uptake by increasing expression of GLUT-4. These effects mandate a down-regulation of insulin secretion.
The resulting reduction of liver insulin activity will suppress liver production of IGF-I while boosting that of IGFBP-1, thereby
decreasing plasma free IGF-I.
Stimulation of AMPK with Metformin also interferes with the
Ras–Raf–MEK–ERK pathway of IGF-I signaling by
inhibiting the ability of IGF-I to activate ras and its downstream targets.
Stimulation of AMPK with Metformin also
blocks the ability of the PI3K-Akt pathway to activate mTOR.
Since the
Ras–Raf–MEK–ERK cascade, as well as
mTOR and its downstream targets, are key mediators of IGF-I’s ability to increase hypertrophy a systemic increase in AMPK activity as brought about by Metformin will hinder the potential for hypertrophy not only by diminishing plasma levels of insulin and free IGF-I, but also by intervening in the post-receptor intracellular pathways mediated events which bring about these bodybuilding effects.





 Reply With Quote
Reply With Quote


